Antifreeze protein - from basic to application - S. Tsuda |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
updated on: 10-OCT-2023 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Antifreeze protein (AFP, including AFGP) is an extraordinary biomolecule that binds to single ice crystal to inhibit its growth (i.e., thermal hysteresis activity) and to lipid bilayer to prolong the cell life time (cell-preservation activity). Prof. Arthur DeVries first discovered AFGP from Antarctic fishes in 1969 [1], then AFP I - III had successively been identified from fishes living in nothern polar sea area till 1984 [2, 3, 4]. Tsuda started AFP exploration in 2000, and has been shown that Japanese organisms also synthesize AFP that are similar but not identical to the polar AFP species {1, 2, 3, 4, 5]. It is interesting that AFP is contained in many edible fishes available in Japanese food markets [6], from which Tsuda prepared the highly purified AFP samples that contain neither cations nor buffer detergents. Insect AFPs [7] and fungal AFPs [8] were also identified from Japanese organisms. Regardless of the difference in primary and tertiary structure, AFPs exhibit the thermal hysteresis and the cell-preservation ability. The following shows the AFPs identified from Japanese organisms. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
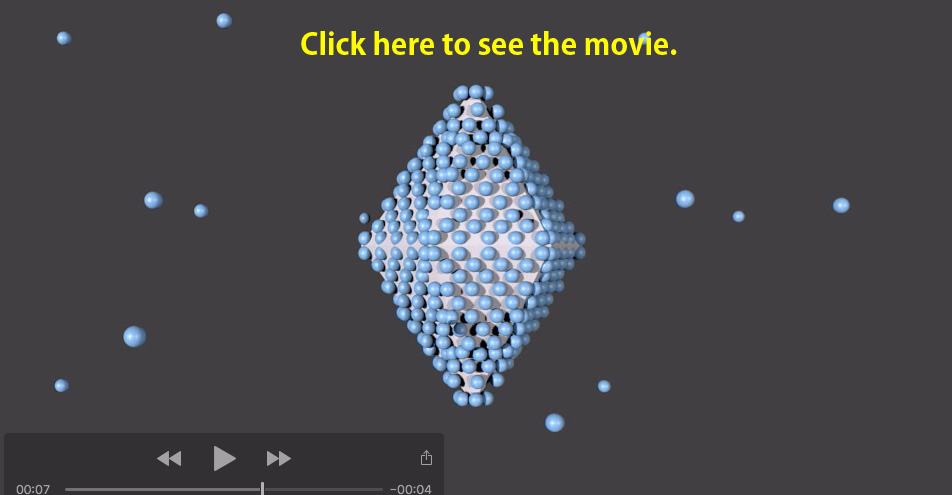
The following movie shows a manner of the binding of numerous fish AFPs onto a single ice crystal. This crystal consists of a hexagonal ice unit/lattice, but takes a rounded shape to minimize the surface energy in water. The lattice while becomes visible as a bipyramid in the presence of AFP, since AFPs bind to waters constructing the prism and/or pyramidal planes of the lattice [2]. In insect and fungal AFPs, the ice is changed into a slightly different morphology (ex. the lemon shape) as they bind to multiple planes including the basal plane. The ice bipyramid is stably observed despite lowering of the temperature. When it is lowered to a certain minus temperature, however, a bursting growth occured from weak points of the ice surface, as they are not sufficiently covered with AFPs (ex. two tips of the bipyramid) [3]. The temperature range, in which the ice is neither grown nor melted, was defined as thermal hysteresis (TH, TH = |Tm - Tf | ). The TH value hence represents the strength of ice-growth inhibition of an AFP molecule [4]. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
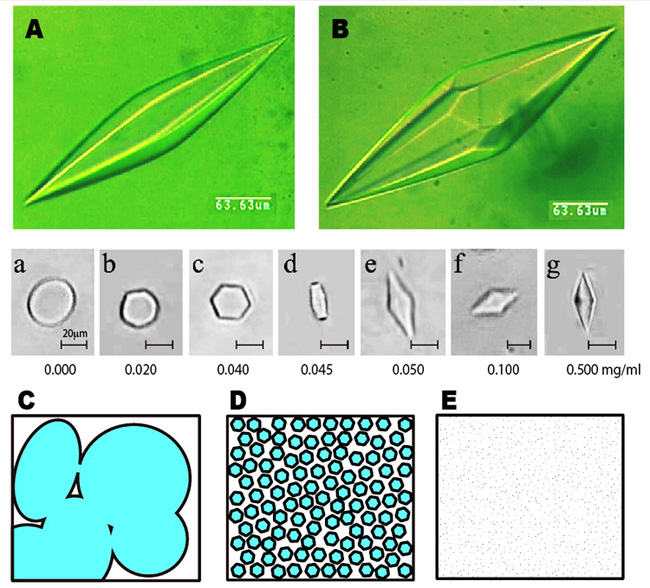
The ice bipyramid created in an AFP solution can be snapshotted on a photomicrometer equipped with a low-temperature controling device. The following A and B show the photo examples, which are named hexagonal bipyramid and hexagonal trapezohedron, respectively. Just one amino acid replacement of AFP is known to modify this crystal shape significantly. When it sets the AFP concentration below 50 ug/ml, the crystal is modified into a rounded hexagonal plate, but not the ice bipyramid (see the snapshots a - g). We call this minimal AFP concentration needed to create ice bipyramid CISC (Critical Ice Shaping Concentration), which should be influenced by ion, suger, glycerol, etc. Now, let us think about the water freezing process. At the moment water is frozen, numerous tiny seed ice crystals are generated in it. They undergo crystal growth and merge to form a multicrystalline state as sketched like C, which is the ordinary ice block that we generally see. This ice block formation while causes the problem if we need quality cryopreservation of water-containing materials, since the ice block expands and physically destroys the inside texture of that materials. The AFP is capable of inhibiting growth of the numerous ice crystals, so that AFP solution in the frozen state is sketched like D. If we can minimize the size of each ice crystal ultimately small, the frozen state will become noncrystalline glass-like state (E). That is, AFP might be able to freeze and preserve the water-containing materials by filling their inside with numerous tiny ice crystals. This can be realized with a home freezer (-20 degC), and does not require liquid nitrogen. The examples of the target mateirials are processed foods, ice cream & frozen sweets, meats, drinks, vegetables, seeds, noodles, gels, inks, polymers, detergents, medicines, etc. However we should take into account that pH, cation/anion, sugar, alcohol, viscosity, and any co-existing substances in the materials may affect/cancel the activity of AFP. We also have to determine CISC very carefully for cryopreservation of the cells and tissues, as the sharpened ice crystal will be harmful. It needs further studies to realize practical use of AFP. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
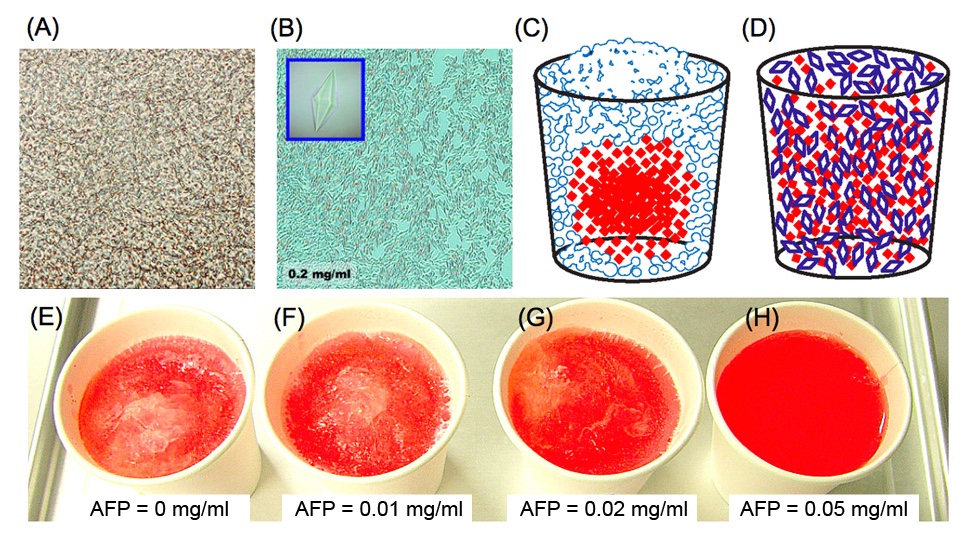
The following shows photomicroscope snapshots of (A) an ordinary ice block and (B) a frozen AFP-solution. The (A) generally contains numerous bubbles, and often snapshotted like this picture. The (A) expands with time and phisically destroys the inside of the frozen material. In contrast, AFP solution becomes an assembly of numerous tiny ice crystals (B) during the freezing storage. Note that ice crystals are shaped into bipyramids in (B) as the AFP concentration is high (0.2 mg/ml). The ice crystals like in (B) are hardly sticked together by AFP-coating, and that they have less power to destroy an inside texture of cryopreserved materials. Such an AFP-induced effect was verified by simple experiments using a colored water. When it freezes the water containing red-colored ink in a home freezer, the inks are gradually excluded from the ice block. This is because ink particles cannot join the ice-crystal-growth that only accepts the waters. The ice block is formed from the portions near the vessel since it is first cooled, and that inks are concentrated into the unfrozen portion (mostly the center of the vessel), as sketched like (C). This is called "freeze-concentration phenomenon" [6]. In contrast, an entirely red-colored ice is created for an AFP solution sketched like (D). The (E) ~ (H) show the snapshots of the frozen colored ice prepared in a home freezer. Each contains 0, 0.01, 0.02, and 0.05 mg/ml of type I AFP, respectively. The efficiency of such "freeze-concentration-inhibition" might be correlated with the TH value of AFP. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
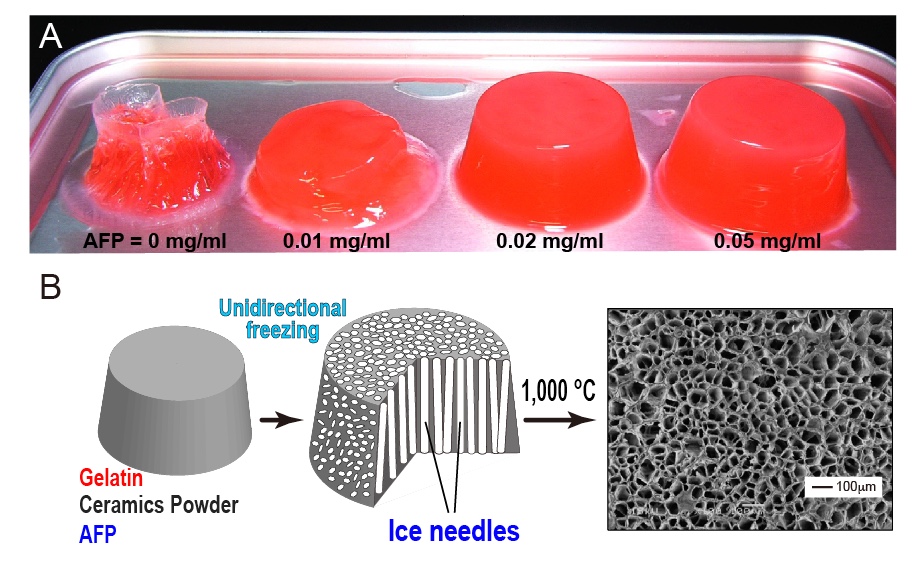
Fabrication of "Feeze-tolerant Gel" is one of the examples of AFP application. The gel consists of polymer network with waters to make a desired viscoelasticity. The jelly, noodle, dough, boilded egg, cakes, and tofu are the well-known examples, which are mostly intorelant to freeze-thawing process, as the freezing induces the ice block formation in the gel to collapse its inside texture. For example, 1-hr freezing storage with a home freezer completely destroyes the structure of 0.5% (w/v) agarose gel (A, left picture), and nulifies its holding ability of waters that flow out concomitantly with thawing. In contrast, the AFP-containing gel is capable of holding its structure (A, 0.01-0.05 mg/ml). In the frozen gel, AFPs will bind onto the ice crystals to inhibit their growth and uniting, leading to preservation of the polymer network during the freeze-thawing process. It is significant only 0.02 mg/ml of AFP is enough for the preservation. The AFP-containing gel is also utilized for creation of "Porous Material", whose examples are the filters, membranes, absorbers, insulators, etc [7]. The first step is to prepare a solution containing gelatin, ceramics powder, and AFP (B, left). After creation of the gel, it is placed on a frozen plate to induce the unidirectional freezing. This procedure forms numerous needle-shaped ice crystals in the frozen gel, and elongates them from bottom to top, without chaging their size and thickness (B, middle). It is difficult to elongate ice needles without AFP. The final step is to sinter the frozen gel containing numerous ice needles at 1,000-1,500 degC. The gelatin and AFP are burning out in this step, while the ceramics are remained to form a porous material that contains extremely fine and unidirectionally aligned dendritic pores (B, right). |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
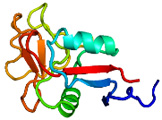
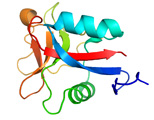
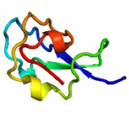
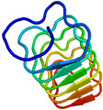
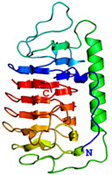
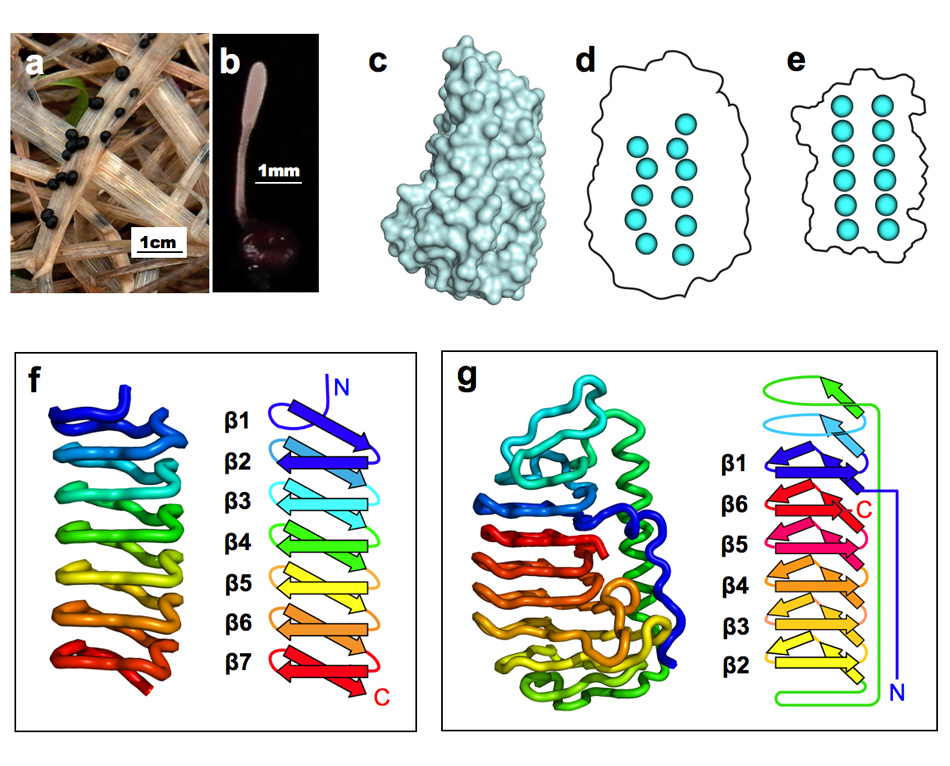
AFP was identified from fishes, insects, plants, bacteria, and fungi in the last 4 decades. We solved the first crystal structure of a fungal AFP, and determined which of its surfaces binds to ice [8]. Our traget was a 223-residue AFP from snow mold fungus,Typhura ishikariensis (photos a and b), which was discovered in 1930s from our place, "a plane of Ishikari" in Hokkaido, Japan. My collaborator (Hoshino, T) characterized the primary sequence of this AFP and named it TisAFP in 2003. It appeared that TisAFP forms a semipear-shaped overall structure as shown in (c), the right side of which is the putative ice-binding site (IBS). The most striking feature of TisAFP was that it can bind to both prism and basal planes of ice crystal similarly to insect AFP, while TisAFP had irregularly arrayed surface-bound waters (d) differently from the insect AFP (f). These waters were thought to share the positions of ice lattice at the moment AFP attaches onto ice. The following (f) and (g) compare the backbone architecture with illustration between insect AFP and TisAFP. The beta-loops are regularly formed from N to C-termini in insect AFP (f). In contrast, the terminal loops are side by side within an irregularly formed parallel beta-helix of TisAFP as illustrated by the spectrum colors showing blue next to red (g). Additionally, the six helical loops of TisAFP are of different lengths. This unique architecture of TisAFP will locate the surface-bound waters irregularly. The structural features of TisAFP may presumably be adopted in the other fungal AFPs. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| a, b: Sclerotium and fruit body of Typhura Ishikariensis, c: Overall structure of an isoform of TisAFP (TisAFP6), d: Illustration of irregularly arrayed waters on the IBS of TisAFP6, e: Illustration of regularly arrayed waters on the IBS of insect AFP, f: Backbone structure of insect AFP consisting of normally ordered beta-helices, g: Backbone structure of TisAFP6 comprising an irregularly ordered beta-helices, in which the N- and C-termini are side by side. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
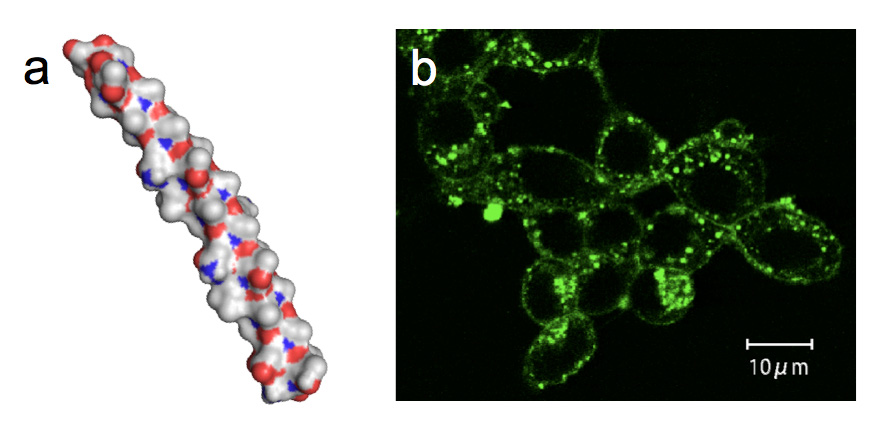
In 1990, Prof. Rubinsky and colleagues in the University of California found that AFPs derived from polar fish serum showed a cell protection function during a hypothermic exposure (+4 degree C). Thereafter, researchers have been performing the hypothermic storage of germ cells, oocytes, and organs with fish AFPs at the temperature just before freezing. However, a scarcity of the fish AFPs that had to be purified from blood sera of polar fishes has been made it difficult to examine such techniques more closely. We found that AFPI-III and AFGP are also expressed in mid-latitude fishes captured in Japan, and their muscle can be utilized as a source material to purify a massive amount of AFP. Here we immersed an insulin production cells (pancreatic islet cell) into a storage solution (Euro-Collins) containing the fish-muscle-derived AFPI-III and AFGP, and examined how the survival rate of the cell changed with preservation time at 4 degree C. Significantly, it was shown that approximately 60% of pancreatic islet cells could alive even after 120 hrs of preservation period in the solution containing 10 mg/mL of AFP that was tentatively categorized into type I [9]. The preserved pancreatic islet cells held the insulin secretion ability. For this type I AFP an alha-helical formation was assumed (Fig. a). A preferable adsorption of the fluorescent type I AFP to the lipid bilayer of the pancreatic islet cells was suggested by a photomicroscope observation (Fig. b). This is consistent with the previous indication that fish AFP exerts the membrane-protection ability. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
If you want to use the AFP samples, please send an e-mail to ST whose address is shown on the top page. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
No reproduction or republication is allowed without permission from ST. |